- 1 (888) 843-5832
- info@dnaconnexions.com
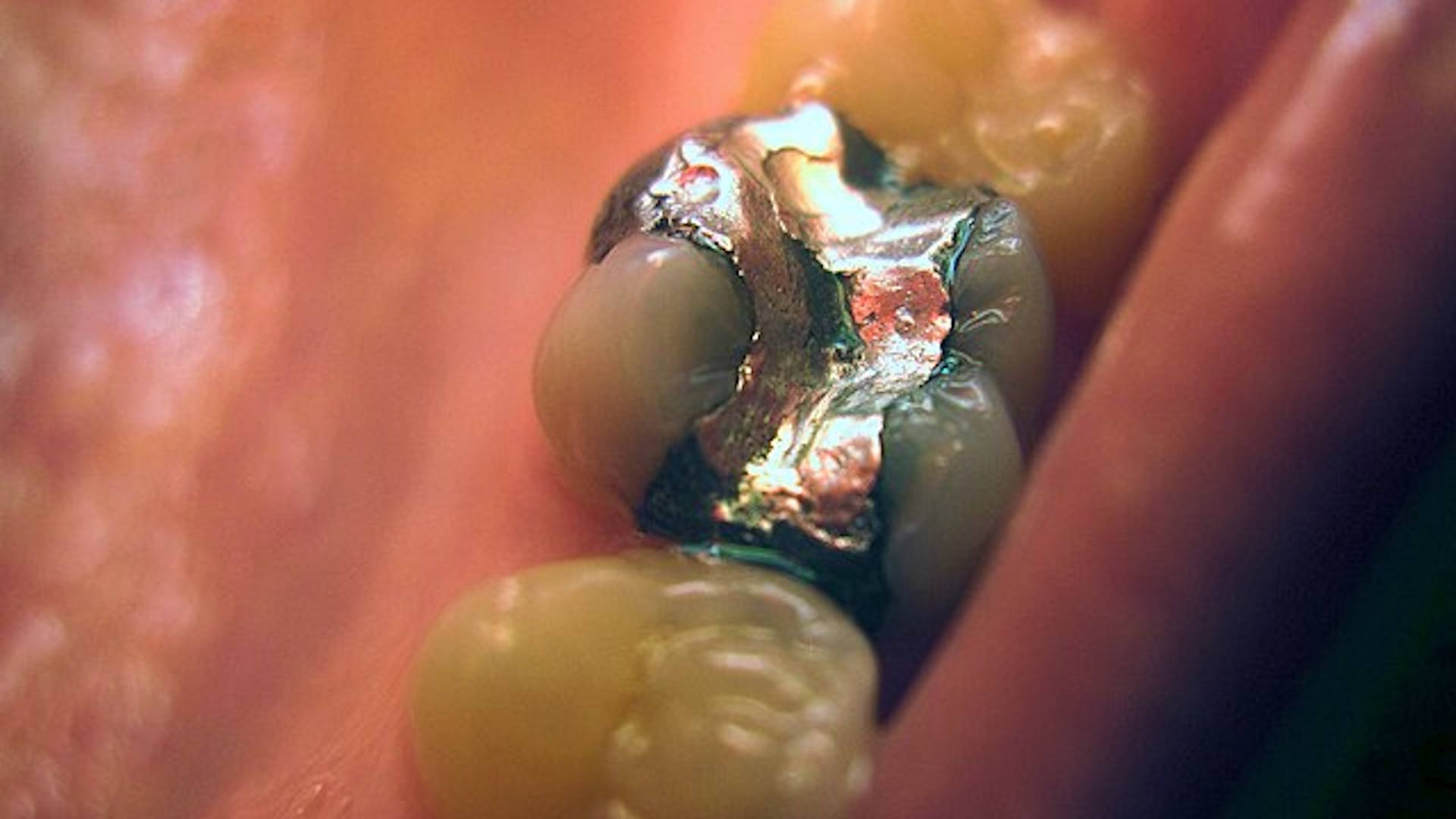
Venice, Florida – In a shocking betrayal of public trust, the U.S. Food and Drug Administration (FDA) has suppressed vital information about Mercury dental amalgam, misleadingly called “silver fillings,” which contain 50% mercury—one of the most toxic non-radioactive substances known.
The FDA refuses to mandate that dentists disclose this danger to patients, while dental boards, influenced by the American Dental Association (ADA)—a trade organization, not a public health authority—punish dentists who reject Mercury use and prioritize safe removal with rigorous informed consent, threatening their licenses. This double-standard is an outrage, leaving millions – – including South Florida’s families, military personnel and veterans – – unaware of the neurotoxin in their mouths.
A groundbreaking 2025 mini-review by Anita Vazquez Tibau, your author, and Blanche D. Grube, DMD, Will the US Food and Drug Administration (FDA) Follow the European Union’s Mercury Dental Amalgam Ban? A Mini-Review, exposes this scandal and demands reform. A new citizen petition calls for immediate action, and Floridians can join the fight by submitting comments at www.regulations.gov.
In 2009, the FDA issued a decision that undermines medical ethics, stating:
“FDA believes that the recommended labeling statements in the special controls guidance document will provide dentists with important information… In addition, FDA notes that dental amalgam is a prescription device and, therefore, patients cannot receive the device without the involvement of a learned intermediary, the dental professional. Based on the reasons described above, FDA has concluded that it is not necessary to require that dentists provide this information to patients, in order to provide reasonable assurance of the safety and effectiveness of the device.” The FDA added: “FDA does not believe it is necessary or appropriate to require that dental health care providers provide this information to patients.”
Meanwhile, dentists who refuse to use Mercury amalgam, and instead focus on safe removal with full informed consent, face harassment, license suspensions or revocations from dental boards influenced by the ADA, which defends Mercury’s use despite its toxicity. Tibau and Grube’s mini-review calls this “scientifically and ethically indefensible,” arguing it conceals a neurotoxin’s risks from patients, including military personnel and veterans who receive amalgam through federal programs.
Mercury dental amalgam releases toxic vapors every moment, with every breath, bite, or swallow, making it a constant threat. International health organizations identify it as the primary source of human Mercury exposure. Tibau and Grube cite National Health and Nutrition Examination Survey data showing over half of Americans over 15 years old have five or more amalgam fillings, with 30–40% exceeding the Environmental Protection Agency’s safety limits. Thousands of studies link Mercury to neurological damage, kidney problems, arthritis, respiratory issues, and antibiotic resistance, a global crisis costing billions. Until around 200 years ago, Mercury was used to cure pelts for making hats and other items of haberdashary. As a result, the expression arose that someone was “Mad as a Hatter!”
Vulnerable groups—pregnant women, children, those with genetic markers like ApoE4 (linked to Alzheimer’s) or CPOX4 (tied to neurobehavioral deficits), and military members and veterans with amalgam fillings—face heightened risks. Autopsy studies show Mercury levels in brain and kidney tissues of amalgam bearers up to 12 times higher, some exceeding toxic thresholds. Recent research connects amalgam to arthritis, with risks peaking at 4–7 fillings, and finds elevated Mercury in women with amalgam. Another study noted neurobehavioral deficits in children with CPOX4 variants, affecting 28% of the population. Yet the FDA claims “little to no information” on risks.
The ADA, a trade organization representing dental industry interests, not public health, has played a disturbing role in perpetuating Mercury’s use. Despite global bans and scientific evidence, the ADA defends amalgam’s cost-effectiveness, influencing the FDA to resist disclosures and a phase-out. This stance is indefensible when safer alternatives like Atraumatic Restorative Treatment (ART) and composite resins are widely used worldwide. The ADA wields sway over dental boards, which punish mercury-free dentists who prioritize patient safety, stifles ethical dentistry and blocks medical freedom.
Tibau and Grube’s mini-review contrasts the FDA’s inaction with global progress. The European Union banned Mercury dental amalgam as of January 1, 2025, aligning with the Minamata Convention on Mercury, which the U.S. ratified in 2013. Over 30 countries, including Norway and Japan, have restricted or banned Mercury dental amalgam. In the U.S., dental clinics contribute about 50% of Mercury in wastewater, polluting Florida’s ecosystems, as noted by the EPA.
In South Florida, local communities, including veterans and military families, face healthcare challenges, worsened by the misleading “silver fillings” label and limited access to Mercury-free options. Military personnel and veterans, often receiving amalgam through Veterans Affairs or military dental programs, face long-term exposure risks. Tibau and Grube argue that the FDA’s reliance on dentists to inform patients is flawed, as many underestimate risks or prioritize cost due to insurance pressures, violating ethical standards, like those set forth in the Declaration of Helsinki, 1964.
Improper removal of Mercury amalgam fillings releases harmful Mercury vapor, endangering patients and dental professionals. Tibau and Grube highlight the Safe Mercury Amalgam Removal Technique (SMART) and PROTECT Protocol, pioneered by Dr. Hal Huggins and advanced by the International Academy of Oral Medicine and Toxicology (IAOMT) and International Academy of Biological Dentistry and Medicine (IABDM). These protocols use high-volume suction, rubber dams, and protective barriers.
Studies confirm high Mercury vapor levels during removal and reduced Mercury levels post-removal with proper methods. The FDA’s failure to promote these safeguards leaves patients, including veterans, and providers at risk.
The FDA’s record is concerning. In 1976, it classified Mercury amalgam as “Generally Recognized as Safe” without rigorous testing. After a citizen petition by attorney James Love, it reclassified amalgam as Class II in 2009, acknowledging risks for vulnerable groups, but rejecting disclosures. A 2010 Scientific Advisory Panel urged warnings, but a 2015 investigation revealed the FDA’s decision was swayed by a cost-benefit analysis favoring economics over health. Another critique noted the FDA’s failure to address cumulative toxicity and antibiotic resistance.
The citizen petition (FDA-2025-P-2526-0001), filed in 2025, demands the FDA ban Mercury dental amalgam, require patient disclosures, and mandate safe removal guidelines to align with the Minamata Convention’s goal to “Make Mercury History.”
Please Follow us on Gab, Minds, Telegram, Rumble, Truth Social, Gettr, Twitter, Youtube
Sources:
• Tibau, A. V., & Grube, B. D. (2025). Will the US FDA Follow the EU’s Mercury Dental Amalgam Ban? A Mini-Review. scivisionpub.com. https://www.scivisionpub.com/previous-display.php?journal=svjoh&&v=9&&i=4&&y=2025&&m=August.
• Citizen Petition, FDA-2025-P-2526-0001. regulations.gov. https://www.regulations.gov/document/FDA-2025-P-2526-0001